Age Dating
There are two main methods determining the age of rocks, relative dating and absolute dating. In the past, relative dating was frequently applied to date age of rocks on the basis of index fossils, geological events, and stratigraphic relationship. Today, as science has developed, more precise radiometric dating methods are applied to determine numerical ages of rocks.
Radiogenic isotope Isotopes are atoms with the same number of protons and electrons but different numbers of neutrons. For example, Carbon (C) have the same atomic number 6, but they have a different number of neutrons, and consequently a different mass number (10C to 16C). 12C and 13C are both stable, that is, they do not spontaneously change their structure and disintegrate. The other five carbon isotopes are radioactive, i.e., they decay spontaneously by the emission of β particles, which are either an electron (β-) or a positron (β+) and are generated from the splitting of a Neutron. In the latter, unstable isotopes are called “radiogenic isotope”. 14C has a long half-life and is a useful isotope.
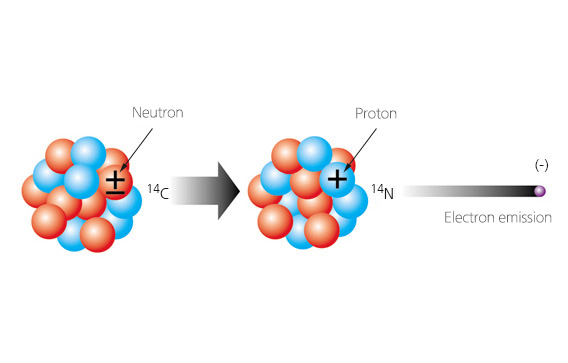
Half life
Radioactive parent elements decay to stable daughter elements. As the parent element decays, its amount decreases while the amount of the daughter element increases. The proportional decay rate for isotopes is expressed as “half-life”, the time it takes for a given amount of a radioactive isotope to be reduced by one-half. For example, in 14C method, parent element is 14C and daughter element is 14N. By knowing how much 14C was originally present in the rock and how much is still there, we can calculate the absolute age of the rock on the basis of its half-life mathematically.
| Radiometric Dating | Decay equation | Half life | Effective dating range(years) |
|---|---|---|---|
| 14C method | 14C → 14N | 5,730 years | 100~40,000 |
| Rb-Sr method | 87Rb → 87Sr | 49 billion years | 10 million~4.6 billion |
| U-Pb method | 238U → 206Pb 235U → 207Pb |
4.5 billion years 700 million years |
10 million~4.6 billion |
| Th-Pb method | 232Th → 208Pb | 14.1 billion years | 10 million~4.6 billion |
| K-Ar method | 40K → 40Ar | 1.3 billion years | 100,000~4.6 billion |
Age of the rockRadiogenic isotopes provide powerful tracers for studying the ages. Those isotopes are contained in various minerals that make up a rock. Radiometric dating, such as 14C, Rb-Sr, U-Pb, Th-Pb and K-Ar method, etc., is a reliable technique in determining the absolute age.

Sphene in Granite
Biotite in Granite
Hornblende in Amphibolite
Muscovite in Gneiss

Monazite (Separated)